Abstract
Background: Overall response rate and response duration in newly diagnosed multiple myeloma (ndMM) has increased in the last decade. However, majority of patients eventually progress and receive multiple lines of therapy. Progression in MM is defined by rise in monoclonal protein and/or clinical manifestations of end-organ damage. However, there is a lack of evidence on the prognostic implication of pattern of progression in the current era. We have hypothesized that patients with clinical manifestations of end-organ damage at first progression have an inferior post-progression overall survival (OS) compared to those with biochemical progression alone.
Method: We evaluated all ndMM patients between 1/1/2008 and 12/31/2015 at the Cleveland Clinic. Key inclusion criterion was patients experiencing first progression requiring an additional line of therapy. Patients with primary refractory disease and those in continued 1st remission at latest follow-up were excluded. Progression was categorized into 2 groups: Biochemical Progression (BP) and Clinical Progression (CP; implying CRAB features). Patients with CP were further stratified based on the presence of extramedullary disease (EM): CP+EM and CP-EM. Progression-free survival (PFS) and OS from first progression were estimated with Kaplan-Meier curves and compared among groups with log-rank test. Cox analysis was used to identify prognostic factors for OS and PFS. Potential prognostic factors included progression pattern, age, gender, race, ISS stage at diagnosis, FISH cytogenetics at diagnosis, metaphase cytogenetics at diagnosis, time from diagnosis to first progression, best response at first remission, frontline autologous stem cell transplant (ASCT), and whether progression occurred while on therapy.
Results: A total of 527 patients with ndMM were evaluated, among which, 257 experiencing 1st progression were included in our analysis. The median age at progression was 64 years. The median time from diagnosis to first progression was 23 months. An autologous stem cell transplantation (ASCT) after induction therapy in 1st remission was performed in 26% of patients. At 1st progression, BP alone was noted in 52% (n=134), CP -EM in 34% (n=87) and CP+EM in 14% (n=36) of patients. In the CP-EM group, the most common mode of progression was development of new bone lesions (76%) followed by anemia (33%), renal insufficiency (18%) and hypercalcemia (13%), with ≥1 mode in approximately one-third of patients. In the CP+EM group, the most common mode of EM progression was development of new plasmacytomas (89%), followed by emergence of circulating plasma cells (14%), with 1 patient having both. A total of 84% of patients progressed on anti-myeloma therapy, which reflects the contemporary practice of continuous therapy in MM. After first progression, 68% received proteasome inhibitors (PIs), 64% received immunomodulatory drugs (IMiDs) and 11% received monoclonal antibodies (MoAbs). Salvage ASCT in 2nd remission was performed in 12% of patients.
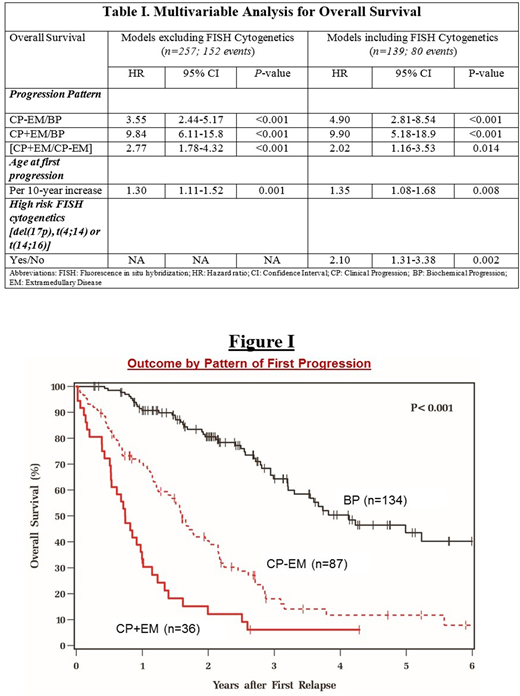
A total of 105 patients (41%) were alive at latest follow-up, with the median follow-up of survivors being 26 months from 1st progression. Median time from diagnosis to 1st progression was shorter in the CP+EM (12 months) compared to CP-EM (25 months) and BP (24 months) groups (P<0.001). There was no significant difference in the progression pattern by depth of 1st remission. The 2-year post-progression OS in BP, CP-EM and CP+EM groups was 81%, 40% and 12% respectively (P<0.001; Figure I). The 2-year post-progression PFS in the respective groups were 35%, 8% and 7% respectively (P<0.001). On multivariable analysis for OS, independent prognostic factors included progression pattern, age at 1st progression and high-risk FISH cytogenetics at diagnosis (Table I).
Conclusion: In the era of PIs, IMiDs and MoAbs, the pattern of 1st progression in MM has a strong and independent prognostic impact on post-progression OS. Patients progressing with clinical manifestations of end-organ damage or extramedullary disease have an inferior PFS and OS compared to those progressing with biochemical criteria alone. These results should be further validated in large prospective studies with data on FISH cytogenetics at relapse. It has clinical implication at the individual level and can also inform the design of clinical trials in relapsed MM.
Majhail:Anthem, Inc.: Consultancy; Incyte: Honoraria; Atara: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.